
As in all nuclear decays, the decaying element (in this case 14 This new element has an unchanged mass number A, but an atomic number Z that is increased by one. In this form of decay, the original element becomes a new chemical element in a process known as nuclear transmutation. Since a proton or neutron has lepton number zero, β + decay (a positron, or antielectron) must be accompanied with an electron neutrino, while β − decay (an electron) must be accompanied by an electron antineutrino.Īn example of electron emission (β − decay) is the decay of carbon-14 into nitrogen-14 with a half-life of about 5,730 years: These particles have lepton number +1, while their antiparticles have lepton number −1. īeta decay conserves a quantum number known as the lepton number, or the number of electrons and their associated neutrinos (other leptons are the muon and tau particles). β + decay is also known as positron emission.
#Beta particles plus
In beta minus (β −) decay, a neutron is converted to a proton, and the process creates an electron and an electron antineutrino while in beta plus (β +) decay, a proton is converted to a neutron and the process creates a positron and an electron neutrino. The two types of beta decay are known as beta minus and beta plus.


Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called positron emission.
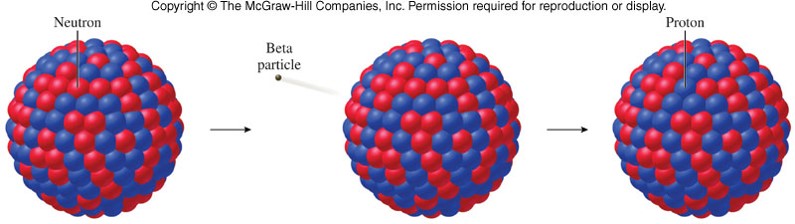
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide.


 0 kommentar(er)
0 kommentar(er)
